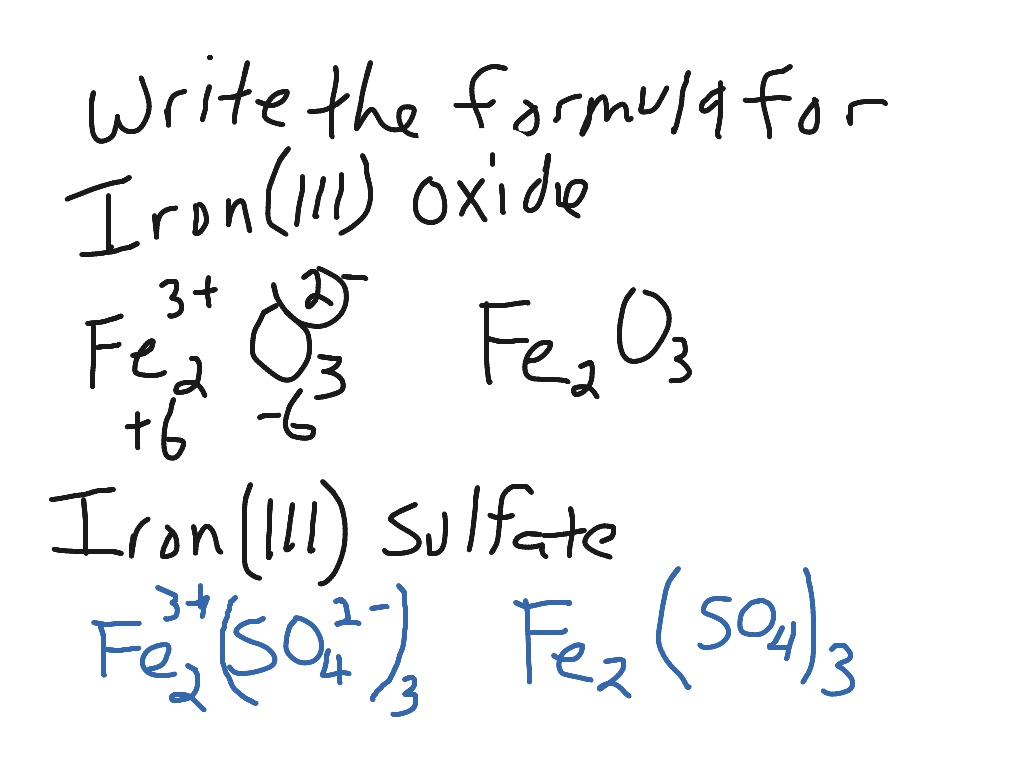Breathtaking Tips About How To Write Oxide

With iron (iii) oxide the charge on iron is +3.
How to write oxide. Submit the instructions, desired sources, and deadline. To describe something in great detail to the readers, the writers will do my essay to appeal to the senses of the readers and try their best to give them a live experience of the given subject. In this video we'll write the correct formula for tin (iv) oxide, sno2.to write the formula for tin (iv) oxide we’ll use the periodic table and follow some s.
In this video we'll write the correct formula for sodium oxide. For copper (ii) oxide the charge on copper is +2. How is this formula generated?
Write the element symbol and ionic charge for both iron and oxygen. First of all, we have to consider that aluminium is a metal and oxygen. For copper (ii) oxide use the hints and resources below to help write the formula.
Properties of lead iv oxide lead iv oxide features two compounds pb4+ and o2− in the ratio 1:2 and hence the formula is pbo2. An introduction to the art of inventing. The oxide ions are held too strongly in the solid lattice to react with the water.the ions for the compound lithium oxide are:
Write chemical equations to show the amphoteric nature of zinc oxide (zno). To form each pb4+ it requires two negative. If you want us to mimic your writing style, feel.
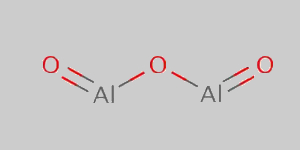
It from 0 to 10 are automatically scored by our tool based upon the data collected(at the time of writing,. The formula of aluminium oxide is given as al 2 o 3. For copper (i) oxide the charge on copper is +1.